Supplements
Benefits and risks of using supplements and sports foods
Group C
Scientific evidence not supportive of benefit amongst athletes OR no research undertaken to guide an informed opinion

Not advocated for use by athletes within Supplement Programs May be permitted for use by identified athletes where there is specific approval from, or reporting to, a Sports Supplement Panel.
The list in this group is identified as “examples” to note and may not be complete.
Supplements
Specific Group C supplements are identified which had previously been classified as Group B. Based on the most recent research, support for their use is less compelling.
Magnesium
Name / Formulation and description:
Magnesium (Mg2+) - as in Magnesium Oxide
Current AIS Supplement Framework Classification: Group C
Agreed AIS Supplement Framework Classification: Group C
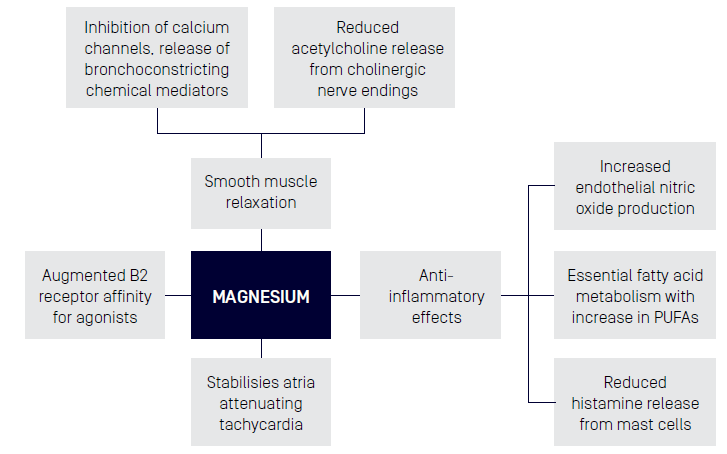
- Anti-inflammatory - Attenuation of the inflammatory IL-6 response1
- Smooth Muscle Relaxant - Reduced blood pressure in the post exercise state2, Bronchodilator effects in Asthma through intravenous or inhaled routes only3
- Neuromuscular strength may be influenced by a higher dose and duration of Magnesium supplementation with training intensity4
- Mood - Magnesium deficiency is thought to contribute to a HPA axis disbalance and associated mood disorders1
- Bone Healing - Magnesium induces an osteogenic effect in the bone marrow space by activating the canonical Wnt signaling pathway, which in turn causes bone marrow stem cells to differentiate toward the osteoblast lineage5
Magnesium plays an important role in many functions in the body. Under normal conditions, dietary intake and GI function, the human body is able to absorb and maintain magnesium at homeostatic levels.
It is thought that magnesium losses through sweat may be greater due to high training volumes in athletes resulting in suboptimal magnesium levels.
Regarding improvements in blood pressure, magnesium plays an important role in muscle contraction and thus correction of deficiency is thought to assist in contract of blood vessels and improvement in blood pressure.
Magnesium and its role in the stress response is not yet clear. Magnesium deficiency is thought to contribute to a HPA axis disbalance and associated mood disorders.1
Overall evidence is equivocal regarding whether Mg supplementation in the realm of RDIs provides a benefit to recovery in athletes. Further exploration of whether any benefit seen is due to suboptimal baseline magnesium levels is needed, as well as larger sample sizes and studies being conducted in both men and women.
Evidence to this point would suggest supplementation does not offer a performance benefit to trained athletes with sufficient dietary intake. Small scale studies suggest it may improve 1RM and countermovement jumps in trained athletes however larger scale studies needed.
Even with consideration for those with suboptimal dietary intake (due to restrictive diets for body composition, due to food beliefs etc.) diet manipulation likely remains the preferred strategy for correction. Given the difficulty in assessing actual Mg status, possible use of a batch tested multivitamin may assist in achieving RDIs if clinical assessment leads to suspicion of suboptimal intake
- Theoretical side effects of toxicity - include hypotension, muscle weakness, respiratory fatigue and apnoea.
- Current evidence would suggest supplementation does not offer a performance benefit to trained athletes with sufficient dietary intake.
- Small scale studies suggest it may improve 1RM and countermovement jumps in trained athletes however larger scale studies needed.
Where nutritional intake is adequate, and no prexisting bony injury, ingestion of addition magnesium may show little benefit to athletes. The consensus of the group was to maintain classification as Group C supplement. It may be included in a “bone pack in conjunction with Calcium and Vitamin D, to complement bone healing in athletes with traumatic or stress fractures.5
- Dmitrašinović, G., Pešić, V., Stanić, D., Plećaš-Solarović, B., Dajak, M., & Ignjatović, S. (2016). ACTH, Cortisol and IL-6 Levels in Athletes following Magnesium Supplementation. J Med Biochem, 35(4), 375-384. doi:10.1515/jomb-2016-0021
- Kass, L., Weekes, J., & Carpenter, L. (2012). Effect of magnesium supplementation on blood pressure: a meta-analysis. Eur J Clin Nutr, 66(4), 411-418. doi:10.1038/ejcn.2012.4
- Bhatnagar, Pallav & Guleria, Randeep & Kukreti, Ritushree. (2006). Pharmacogenomics of ß2-agonist: Key focus on signaling pathways. Pharmacogenomics. 7. 919-33. 10.2217/14622416.7.6.919.
- Heffernan SM, Horner K, De Vito G, Conway GE. The Role of Mineral and Trace Element Supplementation in Exercise and Athletic Performance: A Systematic Review. Nutrients. 2019 Mar 24;11(3):696. doi: 10.3390/nu11030696. PMID: 30909645; PMCID: PMC6471179.
- Chu-Chih Hung et al, The role of magnesium ions in bone regeneration involves the canonical Wnt signaling pathway, Acta Biomaterialia 98 (2019) 246–255
Alpha Lipoic Acid (ALA)
Name / Formulation and description:
Alpha lipoic acid (ALA) is synthesised enzymatically in the mitochondria from octanoic acid and plays a critical role in mitochondrial energy metabolism. ALA presents as two enantiomers: the R-(+) enantiomer, which is widely present in nature and is biologically active, and the S-(-) enantiomer, which is often included in synthetic-based ALA supplements but is believed to have limited biological activity.1
Formulations includes:
- R-alpha lipoic acid capsules, tablets, powders or drops.
- Racemic mixture of R- and S- alpha lipoic acid enantiomers in capsules, tablets, powders or drops.
- Intravenous administration of R-alpha lipoic acid.
Current AIS Supplement Framework Classification: Group C
Agreed AIS Supplement Framework Classification: Group C
- Antioxidant
- Diabetes control*
- Weight loss
*approved for treatment of diabetic neuropathies in Germany2
Antioxidant: ALA acts as an antioxidant through free radical scavenging in vitro.3; 4 However, since ALA only transiently accumulates in tissues in vivo, the significance of direct free radical scavenging activity by ALA in vivo is questionable.5 It is more likely that ALA acts as an indirect antioxidant in vivo that induces or maintains endogenous antioxidant levels.5 ALA can increase glutathione levels within cells.6; 7 ALA can also regenerate reduced vitamin C and vitamin E from their respective oxidized vitamin forms. A pro-oxidant effect of ALA has also been described in experimental studies when relatively high concentrations of ALA are achieved. However, this pro-oxidant effect is believed to occur at levels typically higher than those observed in human studies using oral or intravenous infusion of ALA.8
Diabetic control: Studies that investigated the effects of ALA on diabetes control related to its role in inhibiting glycation reactions and the antioxidant mechanisms of action.
Weight loss: ALA may promote body weight and fat mass reduction via decreasing food intake and enhancing energy expenditure, possibly via suppression of hypothalamic AMP-activated protein kinase (AMPK) activity.9; 10
Sporting/exercise applications: Limited studies in humans show improvements in systemic markers of oxidative stress and antioxidant capacity following muscle-damaging exercise with short-term ALA supplementation11. Evidence from animal studies shows inconclusive effects on skeletal muscle oxidative stress, antioxidant enzymes, mitochondrial biogenesis, and endurance performance.11 Some studies conducted in humans have investigated markers of muscle damage during recovery following an intense muscle-damaging exercise bout with supplementation with ALA (600 mg/day) for 8-10 days.12; 13 Zembron-Lacny et al.13 reported significantly lower creatine kinase following combined submaximal endurance exercise and a muscle damaging eccentric downhill treadmill run, while Zembron-Lacny et al.12 reported no significant effect of ALA supplementation on either creatine kinase or lactate dehydrogenase levels following muscle damaging eccentric resistance exercise.
Diabetes: ALA has been found to reduce micro- and macro-vascular diabetic complications in rodents14; 15 and improve neuropathic pain in rodents16 and humans.2 ALA has also been shown to improve insulin sensitivity in rodents1 and humans17 with diabetes.
Weight loss: a recent meta-analysis of RCTs found a small but significant mean weight loss of 1.27 (95% CI -2.29 to -0.25) kg in clinical patients across studies using doses of 300-1800 mg LA per day for between 8-52 weeks.18
There is an overall lack of studies in humans investigating sporting/exercise-related outcomes, and no conclusive evidence to currently support ALA supplementation for benefits on endurance performance or muscle recovery from intense exercise.
Studies in diabetes are promising, however evidence is mainly limited to rodent data and small, short-term studies in patients with diabetes.
Evidence for weight loss benefits suggest only small weight loss benefits that are arguably not of clinical significance for overweight/obese individuals.
Lacking evidence for improved health or performance in athlete populations.
- Streeper RS, Henriksen EJ, Jacob S, Hokama JY, Fogt DL, Tritschler HJ. (1997). Differential effects of lipoic acid stereoisomers on glucose metabolism in insulin-resistant skeletal muscle. The American journal of physiology, 273, 185-191.
- Mijnhout GS, Kollen BJ, Alkhalaf A, Kleefstra N, Bilo HJ. (2012). Alpha lipoic Acid for symptomatic peripheral neuropathy in patients with diabetes: a meta-analysis of randomized controlled trials. International journal of endocrinology.
- Packer L, Kraemer K, Rimbach G. (2001). Molecular aspects of lipoic acid in the prevention of diabetes complications. Nutrition, 17, 888-895.
- Trujillo M, Radi R. (2002). Peroxynitrite reaction with the reduced and the oxidized forms of lipoic acid: new insights into the reaction of peroxynitrite with thiols. Archives of biochemistry and biophysics, 397, 91-98.
- Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM. (2009). Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochimica et biophysica acta, 1790, 1149-1160.
- Busse E, Zimmer G, Schopohl B, Kornhuber B. (1992). Influence of alpha-lipoic acid on intracellular glutathione in vitro and in vivo. Arzneimittel- Forschung, 42, 829-831.
- Tibullo D, Li Volti G, Giallongo C, Grasso S, Tomassoni D, Anfuso CD, Lupo G, Amenta F, Avola R, Bramanti V. (2017). Biochemical and clinical relevance of alpha lipoic acid: antioxidant and anti-inflammatory activity, molecular pathways and therapeutic potential. Inflammation research: official journal of the European Histamine Research Society, 66, 947-959.
- Gomes MB, Negrato CA. (2014). Alpha-lipoic acid as a pleiotropic compound with potential therapeutic use in diabetes and other chronic diseases. Diabetology & metabolic syndrome, 6, 80.
- Prieto-Hontoria PL, Pérez-Matute P, Fernández-Galilea M, Alfredo Martínez J, Moreno-Aliaga MJ. (2013). Effects of lipoic acid on AMPK and adiponectin in adipose tissue of low- and high-fat-fed rats. European journal of nutrition, 52, 779-787.
- Wang Y, Li X, Guo Y, Chan L, Guan X. (2010). Alpha-Lipoic acid increases energy expenditure by enhancing adenosine monophosphateactivated protein kinase-peroxisome proliferator-activated receptor-gamma coactivator-1alpha signaling in the skeletal muscle of aged mice. Metabolism: clinical and experimental, 59, 967-976.
- Mason SA, Trewin AJ, Parker L, Wadley GD. (2020). Antioxidant supplements and endurance exercise: Current evidence and mechanistic insights. Redox biology, 35, 101471.
- Zembron-Lacny A, Slowinska-Lisowska M, Szygula Z, Witkowski K, Stefaniak T, Dziubek W. (2009). Assessment of the antioxidant effectiveness of alpha-lipoic acid in healthy men exposed to muscle-damaging exercise. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society, 60, 139-143.
- Zembron-Lacny A, Gajewski M, Naczk M, Dziewiecka H, Siatkowski IJJoP, (2013). Physical activity and alpha-lipoic acid modulate inflammatory response through changes in thiol redox status. Biochemistry, 69, 397-404.
- Lin J, Bierhaus A, Bugert P, Dietrich N, Feng Y, Vom Hagen F, Nawroth P, Brownlee M, Hammes HP. (2006). Effect of R-(+)-alpha-lipoic acid on experimental diabetic retinopathy. Diabetologi, 49, 1089-1096.
- Yi X, Maeda N. (206). Alpha-Lipoic acid prevents the increase in atherosclerosis induced by diabetes in apolipoprotein E-deficient mice fed highfat/low-cholesterol diet. Diabetes, 55, 2238-2244.
- Lee WY, Orestes P, Latham J, Naik AK, Nelson MT, Vitko I, Perez-Reyes E, Jevtovic-Todorovic V, Todorovic SM. (2009). Molecular mechanisms of lipoic acid modulation of T-type calcium channels in pain pathway. The Journal of neuroscience : the official journal of the Society for Neuroscience, 29, 9500-9509.
- Jacob S, Ruus P, Hermann R, Tritschler HJ, Maerker E, Renn W, Augustin HJ, Dietze GJ, Rett K. (1999). Oral administration of RAC-alpha-lipoic acid modulates insulin sensitivity in patients with type-2 diabetes mellitus: a placebo-controlled pilot trial. Free radical biology & medicine, 27, 309-314.
- Kucukgoncu S, Zhou E, Lucas KB, Tek C. (2017). Alpha-lipoic acid (ALA) as a supplementation for weight loss: results from a meta analysis of randomized controlled trials. Obes Rev, 18, 594-601.
- Carlson DA, Smith AR, Fischer SJ, Young KL, Packer L. (2007). The plasma pharmacokinetics of R-(+)-lipoic acid administered as sodium R-(+)- lipoate to healthy human subjects. Alternative medicine review : a journal of clinical therapeutic, 12, 343-351.
- Yadav V, Marracci G, Lovera J, Woodward W, Bogardus K, Marquardt W, Shinto L, Morris C, Bourdette D. (2005). Lipoic acid in multiple sclerosis: a pilot study. Multiple sclerosis, 11, 159-165.
- Ziegler D, Hanefeld M, Ruhnau KJ, Hasche H, Lobisch M, Schutte K, Kerum G, Malessa R. (1999). Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: a 7-mon
B-Hydroxy B-Methylbutyrate (HMB)
Name / Formulation and description:
ß-hydroxy ß-methylbutyrate (HMB) is a metabolite of the essential branch chain amino acid leucine, claimed to decrease muscle protein breakdown associated with exercise, increasing muscle mass and strength development associated with resistance training. HMB is also claimed to reduce muscle damage/soreness, enhancing recovery. Much of the initial research on HMB focused on animals, assessing the effect on carcass mass and quality, immune function, morbidity and mortality, colostral milk fat content, growth rates, safety and toxicity. Despite unconvincing results in animal research, HMB supplementation was applied to humans in the mid 1990’s under the presumption that it may enhance gains in muscle size and strength while reducing muscle damage and soreness associated with resistance training and possibly enhance aerobic capacity.1
Two forms of HMB have been used: Calcium HMB (HMB-Ca) and a free acid form of HMB (HMB-FA). HMB-FA may increase plasma absorption and retention of HMB to a greater extent than HMB-CA. However, research with HMB-FA is in its infancy, and there is not enough research to support whether one form is superior.
Current AIS Supplement Framework Classification: Group B (Other)
Agreed AIS Supplement Framework Classification: Group C
- Enhanced skeletal muscle hypertrophy & strength adaptation in response to resistance exercise.
- Mitigation of exercise induced muscle damage and/or enhanced recovery following strenuous exercise.
- Augment acute immune, inflammatory and endocrine responses following exercise.
- Prevention of skeletal muscle disuse atrophy.
HMB induces acute muscle anabolism via increased in muscle protein synthesis (MPS) and reduced muscle protein breakdown (MPB).2
While there is evidence to show acute HMB supplementation does stimulate MPS and moderate MPB, the effect is less than leucine ingestion alone and significantly less than acute whey protein ingestion.2 Acute HMB supplementation does not appear to influence serum testosterone and cortisol levels3 , nor indices of inflammation, such as C-reactive protein.4 There is preliminary evidence suggesting potential benefit in mitigating disuse atrophy, at least in older individuals.5
When contrasted against accepted dietary interventions like post-exercise whey protein ingestion, HMB does not appear to further augment the response.6 Concerns have been raised about the integrity of recently published data on HMB supplementation across a 12-week training period, given the significance of the response.7
While there is some evidence supporting the claims that HMB supplementation favourably influences skeletal muscle protein metabolism, efficacy is significantly less than that of leucine alone, and much less than acute ingestion of high biological value proteins. As such, these more efficacious interventions should be prioritised over HMB.
- Slater GJ, Logan PA, Boston T, Gore CJ, Stenhouse A, Hahn AG. Beta-hydroxy beta-methylbutyrate (HMB) supplementation does not influence the urinary testosterone: epitestosterone ratio in healthy males. J Sci Med Sport. 2000 Mar;3(1):79-83.
- Wilkinson DJ, Hossain T, Hill DS, Phillips BE, Crossland H, Williams J, Loughna P, Churchward-Venne TA, Breen L, Phillips SM, Etheridge T, Rathmacher JA, Smith K, Szewczyk NJ, Atherton PJ. Effects of leucine and its metabolite ß-hydroxy-ß-methylbutyrate on human skeletal muscle protein metabolism. J Physiol. 2013 Jun 1;591(11):2911-23.
- Teixeira FJ, Matias CN, Monteiro CP, Valamatos MJ, Reis JF, Tavares F, Batista A, Domingos C, Alves F, Sardinha LB, Phillips SM. Leucine Metabolites Do Not Enhance Training-induced Performance or Muscle Thickness. Med Sci Sports Exerc. 2019 Jan;51(1):56-64.
- Wilson JM, Lowery RP, Joy JM, Walters JA, Baier SM, Fuller JC Jr, Stout JR, Norton LE, Sikorski EM, Wilson SM, Duncan NM, Zanchi NE, Rathmacher J. ß-Hydroxy-ß-methylbutyrate free acid reduces markers of exercise-induced muscle damage and improves recovery in resistance-trained men. Br J Nutr. 2013 Aug 28;110(3):538-44
- Deutz NE, Pereira SL, Hays NP, Oliver JS, Edens NK, Evans CM, Wolfe RR. Effect of beta-hydroxy-beta-methylbutyrate (HMB) on lean body mass during 10 days of bed rest in older adults. Clin Nutr. 2013 Oct;32(5):704-12.
- Jakubowski JS, Wong EPT, Nunes EA, Noguchi KS, Vandeweerd JK, Murphy KT, Morton RW, McGlory C, Phillips SM. Equivalent Hypertrophy and Strength Gains in β-Hydroxy-β-Methylbutyrate- or Leucine-supplemented Men. Med Sci Sports Exerc. 2019 Jan;51(1):65-74.
- Wilson JM, Lowery RP, Joy JM, Andersen JC, Wilson SM, Stout JR, Duncan N, Fuller JC, Baier SM, Naimo MA, Rathmacher J. The effects of 12 weeks of beta-hydroxy-beta-methylbutyrate free acid supplementation on muscle mass, strength, and power in resistance-trained individuals: a randomized, double-blind, placebo-controlled study. Eur J Appl Physiol. 2014 Jun;114(6):1217-27.
Branched-chain amino acids (BCAA)
Name / Formulation and description:
Branched chain amino acids (BCAA i.e. leucine, isoluceine and valine usually in a 2:1:1 ratio) and leucine in isolation are purified amino acids appearing as crystalline powders. They are poorly soluble in water and bitter tasting. The sources of the protein from which BCAA/ LEU are derived from are not immediately apparent on the packaging of many products. However, there are several sources1:
- Purified from processed animal feathers/fur/hair/skin
- Purified from processed plant proteins
- Fermented by genetically modified micro-organisms engineered to ferment sugar to the amino acids in question.
There is some concern that they may also be derived from human hair.1
Current AIS Supplement Framework Classification: Group B
Agreed AIS Supplement Framework Classification: Group C
- Enhanced endurance capacity/reduced fatigue.
- Improved recovery following muscle damage (reduced soreness/damage).
- Increased muscle mass via activating muscle protein synthesis.
BCAAs are essential amino acids metabolised primarily within the skeletal muscle and they play an important role in both cellular energy homeostasis2 and in the regulation of muscle protein synthesis.3 Theoretically, by supporting energy metabolism and by stimulating muscle protein synthesis it is suggested that BCAA/ leucine may support muscle growth. Furthermore, there is some evidence that BCAA/leucine supplementation may assist in the recovery from muscle damaging exercise via similar mechanisms to those described above.
Finally, BCAA supplementation may provide substrate to working muscle under glycogen depleted conditions and due to BCAA competition for transport into the brain with tryptophan, BCAA supplementation may limit tryptophan entry into the brain. Theoretically this would reduce serotonin production in the brain and limit the onset of fatigue.4
The evidence for BCAA/leucine supplementation supporting endurance performance is equivocal.4 A recent study5 suggests that 20 g of BCAA ingested 1hr prior to a ramp test on a treadmill can delay fatigue. However, like much of the BCAA literature around endurance performance/fatigue there are significant flaws in the research. In the case of 5 the placebo is not matched for calories. A thorough analysis of the most recent research on BCAA and endurance performance is needed to determine their efficacy for promoting performance. There is a great deal of heterogeneity in the literature around supplementation protocols and dosing strategies and so firm recommendations on this are difficult. There is an argument however, that because BCAAs become significant substrates during prolonged exercise that supplementing may prevent muscle damage/breakdown, but there is little evidence to support this notion. For instance, 20 g of BCAA supplemented before and during a 100 km race (3 g 1hr before followed by 17 g throughout a 100 km race) did not affect performance or markers of muscle damage.6
There is building evidence for BCAA supplementation to augment the response to damaging exercise. A meta-analysis of the literature from 2007-2013 suggests that BCAA supplementation may significantly reduce the severity of delayed onset muscle soreness following damaging exercise when compared to placebo treatments.7 Additionally, a systematic literature review carried out on research published up to August 2017 suggests that there may be a modest benefit of BCAA supplementation for markers of muscle damage.8 In an analysis of the dosing strategies in this systematic review8 it is suggested that a daily intake greater than 200 mg*kg*day-1 (~16 g) for at least 7 days prior to the damaging exercise may alleviate some of the impacts of muscle damage on muscle performance (force decrement, plasma CK). However, it should be noted that the placebo in all the included trials is devoid of any protein. So, it remains to be seen if BCAA supplementation would be better/worse than intact protein for this outcome measure. Furthermore, the systematic review suggests efficacy of BCAA supplementation only if the damage is low-moderate.
Because BCAA/leucine are critical for signalling to increase muscle protein synthesis3, it has long been thought that BCAA/leucine supplementation may enhance muscle protein synthesis and therefore growth in response to nutrition/exercise. However, the case for supplementing BCAA/leucine in isolation seems to be weak at best. For instance, when 5.6 g (equivalent content in 20 g of Whey) of BCAA were supplemented following a session of resistance exercise the resulting increase in muscle protein synthesis was only 22%.8 With intact protein we would expect this stimulatory response on muscle protein synthesis to be at least double that. So, whilst BCAAs, when taken in isolation following resistance exercise, can stimulate muscle protein synthesis they probably should not be recommended over whole foods containing sufficient high-quality protein. However, there may be a case for utilising BCAA/leucine to “top up” the anabolic potential of sub-optimal meals. The leucine content of a meal seems to be the key driver of the anabolic response (muscle protein synthesis) to that meal. Approximately 2.5 g of leucine per meal (equivalent to ~20 g of whey protein) seems to be sufficient to maximise muscle protein synthesis.3 Furthermore, when a suboptimal dose of whey protein (6.25 g whey, 0.75 g of leucine) in a mixed macronutrient beverage is “topped up” with leucine to contain 3 g of leucine, it produces a similar muscle protein synthesis response in the recovery from resistance exercise as 25 g of whey protein (3g of leucine).9 These data suggest that leucine could be used to enhance the anabolic potential of certain meals that may not, on their own, maximise muscle protein synthesis. This could take the form of supplementing sub-optimal meals (plant-based meals, meals with less than 20-30 g of high-quality protein) with up to 3 g of additional leucine. However, we do not know if this strategy would support muscle growth in the long term.
Endurance performance: The evidence on the role of BCAA/leucine in supporting endurance performance or preventing damage from long duration activity is very heterogeneous and equivocal. From the literature so far, a clear dosing strategy cannot reliably be suggested especially considering that the placebo is often not optimal for assessing outcomes. There is probably room in the literature for a systematic review/meta-analysis to address this aspect of BCAA/Leucine supplementation.
Muscle damage recovery: The evidence for BCAA supplementation in reducing the severity of symptoms following muscle damage protocols (drop jumps, repeated eccentric contractions) is building. But the benefits appear to be marginal and given that the placebo is often simply a calorically matched product devoid of protein it would be hard to argue for the supplementation protocol to be implemented in place of a sound diet with sufficient high quality protein. Furthermore, the supplementation protocols that seem to be effective (when compared against placebos containing no protein) are likely impractical given the large daily doses required (16-20 g).
Muscle anabolism: Where intact and high-quality protein can be consumed in sufficient quantities to maximise muscle anabolism there appears to be little-no need to supplement with BCAA/Leucine. However, where a meal is going to be sub-optimal for maximising muscle anabolism (plant based protein or less than 20-30 g of high quality protein) then there may be a benefit to supplementing that meal with leucine up to a total of 2.5-3 g of leucine. It should be noted that the “benefit” in this instance is purely for muscle protein synthesis and there is not yet firm evidence of this kind of dosing strategy augmenting other outcomes such as muscle growth, strength, or recovery in athletic populations.
While there is some evidence supporting the claims that BCAA/leucine supplementation favourably influences skeletal muscle protein metabolism, efficacy is significantly less than acute ingestion of high biological value proteins. As such, these more efficacious interventions should be prioritised over BCAA/leucine. The fortification of lower biological value proteins with additional BCAA/leucine to optimise muscle anabolism is recognised and discussed in a separate fact sheet on isolated protein supplements.
- Huang J., Nkrumah P.N., Appiah-Sefah G., Tang S. (2013). Authentication of pure L-Leucine products manufactured in China by discriminating between plant and animal sources using nitrogen stable isotope technique. Journal of Food Science, 78, 490 – P494.
- Kainulainen H., Hulmi J., Kujala U. (2013). Potential role of branched-chain amino acid catabolism in regulating fat oxidation. Exercise and Sport Science Reviews, 41(4), 194-200.
- Gorissen S., and Phillips S. (2019). Branched-Chain Amino Acids (Leucine, Isoleucine, and Valine) and Skeletal Muscle. Nutrition and Skeletal Muscle, 16, 263-279.
- Williams M. (2005). Dietary Supplements and Sports Performance: Amino Acids. Journal of the International Society of Sports Nutrition, 2(2), 63-67.
- AbuMoh’d M., Matalgah L., and Al-Abdulla Z. (2020). Effects of Oral Branched-Chain Aino Acids (BCAAs) Intake on Muscular and Central Fatigue During an Incremental Exercise. Journal of Human Kinetics, 31(72), 69-78.
- Knechtle B., Mrazek C., Wirth A., Knechtle P., Rust C., Senn O., Rosemann T., Imoberdorf R., Ballmer P. (2012). Brahcned-Chain Amino Acid Supplementation during a 100km Ultra-Marathon – A Randomized Controlled Trial. J Nutr Sci Vitaminol, 58, 36-44.
- Fedawa M., Spencer S., Williams T., Becker Z and Fuqua C. (2019). Effect of Branced-Chain Amino Acid Supplementation on Muscle Soreness Following Exercise: A Meta-Analysis. International Journal for Vitamin and Nutrition Research, 89, 348-356.
- Jackman S., Witard., Philp A., Wallis G., Baar K., Tipton K. (2017). Branched-Chain Amino Acid Ingestion Stimulates Muscle Myofibrillar Protein Synthesis Following Resistance Exercise in Humans. Frontiers in Physiology.
- Churchward-Venne T., Breen L., Di Donato D., Hector A., Mitchell C., Moore D., Stellingwerff T., Breuille D., Offord E., Baker S., Phillips S. (2014). Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: a double-blind randomized trial. American Journal of Clinical Nutrition, 99(2), 276-86.
Phosphate
(Phosphorus)
Name / Formulation and description:
Phosphorus is a non-metallic essential nutrient, with about 11–14 g phosphorus per kg of fat-free mass (FFM) stored in the human body. Of which ~85% is located in the skeletal system. Comes in three forms, including sodium, calcium & potassium phosphate. However, most research is on sodium phosphate.
Current AIS Supplement Framework Classification: Group B (Other)
Agreed AIS Supplement Framework Classification: Group C
Current investigations of phosphate supplementation have focused on the physiological and performance-related outcomes of laboratory protocols including graded exercise tests to exhaustion, the 30-s Wingate test, 6 × 20 m (~3– 4 s) repeat sprint efforts, and TT situations ranging in duration from 3–60 min. Overall, there is equivocal evidence of performance enhancement from phosphate supplementation.1
Proposed benefits include:
- Increased aerobic capacity.
- Increased peak power output.
- Increased anaerobic threshold.
- Improved myocardial and cardiovascular responses to exercise.
The proposed mechanisms underpinning these benefits include an enhanced rate of ATP and PCr resynthesis; improved buffering capacity to support high rates of anaerobic glycolysis; improvement of myocardial contractility leading to increased cardiac efficiency; and an increased erythrocyte 2,3 diphosphoglycerate 2,3 DPG concentration, leading to a reduced affinity of oxygen with haemoglobin and a greater unloading of oxygen to the peripheral tissues.
In some instances, phosphate has been shown to enhance VO2max 2, 3, anaerobic threshold3, and cycling TT performance.4 However, in the case of repeated sprints, the magnitude of benefit has been shown to be varied and unclear.5 Finally, there is also a large amount of contrary evidence from the same physiological and performance measures that suggests phosphate supplementation (in isolation, or in combination with other buffer agents) has no impact on exercise capacity or performance outcomes.6, 7, 8, 9
Current evidence regarding the efficacy of phosphate supplementation remains unclear, since there exists no evidence to suggest an accumulation of this supplement in the muscle, where a number of the reported mechanism are suggested to take effect.
Typically, phosphate supplementation is achieved over a 3–6 day period, with a total daily dose of ~50 mg/kg of fat-free mass (~3–5 g/ day) consumed in single or split doses throughout the day. This is often associated with GI distress.2, 9 However, tolerance is improved by concurrent consumption with ~300 ml of a carbohydrate-rich fluid.10
The use of this supplement for enhanced athletic performance is likely questionable, with further research needed to fully explore its true effect.
- Peeling, Binnie, Goods, Sim & Burke. (2018). Evidence based supplements for the enhancement of athletic performance. Int J Sport Nutr Exerc Metab, 28, 178-87.
- Cade, Conte, Zauner, Mars, Peterson,, Lunne, & Packer. (1984). Effects of phosphate loading on 2, 3-diphosphoglycerate and maximal oxygen uptake. Med Sci Sports Exerc, 16, 263-268.
- Kreider, Miller, Williams, Somma, & Nasser. (1990). Effects of phosphate loading on oxygen uptake, ventilatory anaerobic threshold, and run performance. Medicine & Science in Sports & Exercise, 22(2), 250–256.
- Folland, Stern & Brickley. (2008). Sodium phosphate loading improves laboratory cycling time-trial performance in trained cyclists. J Sci Med Sport, 11, 464–468.
- Kopec, Dawson, Buck & Wallman. (2016). Effects of sodium phosphate and caffeine ingestion on repeated-sprint ability in male athletes. J Sci Med Sport, 19, 272-276.
- Brewer, Dawson, Wallman & Guelfi. (2014). Effect of sodium phosphate supplementation on cycling time trial performance and VO2 1 and 8 days post loading. J Sports Sci Med, 13, 529–534.
- Goss, Robertson, Riechman, Zoeller, Dabayebeh, Moyna & Metz. (2001). Effect of potassium phosphate supplementation on perceptual and physiological responses to maximal graded exercise. Int J Sport Nutr Exer Metab, 11, 53–62.
- Kraemer, Gordon, Lynch, Pop & Clark. (1995). Effects of multibuffer supplementation on acid-base balance and 2, 3-diphosphoglycerate following repetitive anaerobic exercise. Int J Sports Nutr, 5, 300–314.
- West, Ayton, Wallman & Guelfi. (2012). The effect of 6 days of sodium phosphate supplementation on appetite, energy intake, and aerobic capacity in trained men and women. Int J Sport Nutr Exerc Metab, 22, 422-429.
- Brewer, Dawson, Wallman & Guelfi. (2013). Effect of repeated sodium phosphate loading on cycling time-trial performance and VO2peak. Int J Sport Nutr Exerc Metab, 23, 187-194.
Prebiotics
Name / Formulation and description:
Prebiotics: A prebiotic is defined as ‘a substrate that is selectively utilized by host microorganisms conferring a health benefit.’1
Sources include ‘prebiotic-like’ foods and products that contain (one or more) concentrated extracts. Fructo -oligosaccharides (FOS), galacto-oligosaccharides (GOS), soybean- oligosaccharides, mannan oligosaccharides, xylo-oligosaccharides, inulin, partially hydrolysed guar gum, lactulose, and resistant starches (types 1, 2, 3, and 4).
The doses used in studies demonstrating a significant alteration in microbial composition and metabolism, vary greatly and are individual-microbiome dependent.
Current AIS Supplement Framework Classification: N/A
Agreed AIS Supplement Framework Classification: Group C
Prebiotics are substrates selectively utilised by the host microbiome conferring a health benefits to the host. They may:
- Enhance gastrointestinal immune function and competitive inhibition of pathogens (help an athlete be more resistant to infections i.e., traveller’s diarrhoea2 and UTIs)
- Improve bowel function3
- Have some anti-colon cancer properties3
- Have some lipid lowering effects4
- Improve glucose tolerance4
Consumption of prebiotic like foods and/or prebiotic supplements balance and restore a dysbiotic gastrointestinal environment caused by negative factors including diet, certain medication use, physical and psychological stressors.
While complex, it is thought they do this by increasing microbial diversity and key bacterial species that produce a variety of end products including short chain fatty acids.
Prebiotic mechanisms:
- increase Bifidobacterium spp.
- increase lactobacillus spp.
- increase faecalibacterium prausnitzi,
- increase in Roseburia spp.
- increase in Eubacterium spp.
- decrease beta-glucuronidase activity
- decrease faecal concentrations of protein putrefactive by products and pathogenic bacteria
Most human studies evaluating the effects of prebiotics have explored effects on the composition of the gastrointestinal microbiota. These studies report significant increases in Bifidobacterium and to a lesser-degree Lactobacillus bacterium and Faecalibacterium prausnitzi, Roseburia and Eubacterium spp. and their by-products.
The main prebiotics researched are fructo-oligosaccharides (FOS), galacto-oligosaccharides (GOS), lactulose and partially- hydrolysed guar gum.
Taking Lactulose, GOS and FOS have all been found to be effective in the management of constipation. GOS have been found to reduce the incidence of ‘traveller’s diarrhoea and FOS to reduce the severity of diarrhoea.
Administration of lactulose or oligofructose-enriched inulin has been found to reduce beta-glucuronidase activity (protective against colon cancer).
Lactulose has been shown to reduce incidence of urinary tract infections.
Inulin plus oligofructose has been shown to significantly improve calcium absorption in women. and promote satiety
- Prebiotic foods and ingredients used in products are considered safe.
- The ‘capacity’ of a prebiotic ingredient to exert an effect on the microbial ecosystem is dependent on the microbes present in the gut to ferment them.
- The main adverse effects are gastrointestinal symptoms of bloating, distension, and increased flatulence.
- Effect on sport performance is varied, because of a high degree of inter-individual variation in response.
- There remain some conflicting results regarding therapeutic outcomes e.g., FOS has not shown benefits in reducing symptoms of irritable bowel syndrome but GOS has.
- Allergies to prebiotics ingredients are rare but have been reported.
Group C
- Gibson, G. R. et al. (2017). Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nature reviews Gastroenterology & Hepatology, 14, 491.
- De Preter, V. et al. (2008). Effect of dietary intervention with different pre-and probiotics on intestinal bacterial enzyme activities. European Journal of Clinical Nutrition, 62, 225-231.
- Vulevic, J., Juric, A., Tzortzis, G. & Gibson, G. R. (2013). A mixture of trans-galactooligosaccharides reduces markers of metabolic syndrome and modulates the fecal microbiota and immune function of overweight adults. The Journal of Nutrition, 143, 324-331.
- Cummings, J., Christie, S. & Cole, T. (2001). A study of fructooligosaccharides in the prevention of travellers’ diarrhoea. Alimentary Pharmacology & Therapeutics, 15, 1139-1145.
Vitamin E
Name / Formulation and description:
Vitamin E refers to lipid soluble compounds including four tocopherols and four tocotrienols, with α-tocopherol being the most biologically available and most well-known form. It is found in lipid rich structures such as the sarcoplasmic reticulum, where it scavenges free radicals produced by the mitochondria, thereby reducing lipid peroxidation and membrane damage.
Given Vitamin E is a fat-soluble vitamin, it is primarily found in higher fat, plant derived foods, including nuts and oils, and to a lesser extent in the fats of meat, poultry and fish. Both natural and synthetic forms of vitamin E can be found in vitamin E supplements, with the “d” prefix denoting natural forms, particularly d-alpha tocopherol; and the “dl” prefix denoting synthetic forms, particularly dl-alpha tocopheryl acetate. Vitamin E supplements are available in the form of tablets, capsules, powders or drops.
Current AIS Supplement Framework Classification: Group B
Agreed AIS Supplement Framework Classification: Group C
- Reduction in oxidative stress and free radical damage induced by intense exercise
- Enhanced recovery following fatiguing exercise
Vitamin E appears to have a role in immune function, including increasing lymphocyte proliferation in response to mitogenic stimulation, increasing interleukin-2 production, decreasing interleukin-6 production and enhanced delayed type hypersensitivity response.1 Vitamin E has anti-oxidant properties, and is capable of scavenging lipidderived peroxyl radicals and terminating oxidation of polyunsaturated fatty acids.2,3
Overall, there is limited evidence to support the use of vitamin E for athletes.
- Studies are not supportive of any benefit of vitamin E supplementation on rate of recovery of muscle contraction force following fatiguing exercise in humans4-6
- Despite evidence for improvements in oxidative stress with vitamin E supplementation in athletes/exercisers, its effects on exercise performance outcomes are not convincing7
- Two studies found improvements in cycling anaerobic threshold power during incremental tests at altitude following vitamin E supplementation, however both studies were small in size8-9
Some studies have shown improvements in non-fatal myocardial infarction risk in patients with existing heart disease following vitamin E supplementation, however RCTs are generally not supportive of any benefits of vitamin E supplements in the primary or secondary prevention of cardiovascular disease.10
Some investigations have shown a potential blunting of skeletal muscle adaptations to endurance training with combined vitamin E and vitamin C supplementation.11-13 There is currently no convincing evidence that vitamin E supplements alone impair exercise-related adaptations in humans. While vitamin E is generally considered ‘safe’ even with intake well above RDI14, a meta-analysis of clinical trials of vitamin E supplementation across a range
of different clinical conditions reported that adults who consume ≥400 IU/day were 6% more likely to die of any cause compared to no supplementation.15 Other meta-analyses of human RCTs have found that there is no risk of increased CVD mortality or all-cause mortality at doses of up to 800 IU/day.16-17
Chronic supplementation with vitamin E amongst athletic populations can not currently be supported. However, it is recognised that further research in this area is warranted. This includes research into the potential acute benefits of supplementation when immediate performance retention is desired, and adaptation is less important, such as during competition.
If moderating oxidative stress and inflammation are a priority, adapting a meal plan that focuses on unprocessed, conventional foods to include additional serves of mixed fruit and vegetables, plus nuts and extra virgin plant based oils should be a priority.
- Lee GY, Han SN. (2018). The Role of Vitamin E in Immunity. Nutrients, 10, 1614.
- Traber MG, Atkinson J. (2007). Vitamin E, antioxidant and nothing more. Free radical biology & medicine, 43, 4-15.
- Forman HJ, Davies KJ, Ursini F. (2014). How do nutritional antioxidants really work: nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free radical biology & medicine, 66, 24-35.
- Jakeman P, Maxwell S. (1993). Effect of antioxidant vitamin supplementation on muscle function after eccentric exercise. European journal of applied physiology and occupational physiology, 67, 426-430.
- Beaton LJ, Allan DA, Tarnopolsky MA, Tiidus PM, Phillips SM. (2002). Contraction-induced muscle damage is unaffected by vitamin E supplementation. Medicine and science in sports and exercise, 34, 798-805.
- Helgheim I, Hetland O, Nilsson S, Ingjer F, Stromme SB. (1979). The effects of vitamin E on serum enzyme levels following heavy exercise. European journal of applied physiology and occupational physiology, 40, 283-289.
- Mason SA, Trewin AJ, Parker L, Wadley GD. (2020). Antioxidant supplements and endurance exercise: Current evidence and mechanistic insights. Redox biology, 35, 101471.
- Simon-Schnass I, Pabst H. Influence of vitamin E on physical performance. (1988). International journal for vitamin and nutrition research Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung Journal international de vitaminologie et de nutrition, 58, 49-54.
- Kobayaski Y. (1974). Effect of vitamin E on aerobic work performance in man during acute exposure to hypoxic hypoxia.University of New Mexico.
- Saremi A, Arora R. (2010). Vitamin E and cardiovascular disease. American journal of therapeutics, 17, 56-65.
- Paulsen G, Cumming KT, Holden G, Hallen J, Ronnestad BR, Sveen O, Skaug A, Paur I, Bastani NE, Ostgaard HN, Buer C, Midttun M, Freuchen F, Wiig H, Ulseth ET, Garthe I, Blomhoff R, Benestad HB, Raastad T. (2014). Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: a double-blind, randomised, controlled trial. The Journal of physiology, 592, 1887-1901.
- Morrison D, Hughes J, Della Gatta PA, Mason S, Lamon S, Russell AP, Wadley GD. (2015). Vitamin C and E supplementation prevents some of the cellular adaptations to endurance-training in humans. Free radical biology & medicine, 89, 852-862.
- Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Bluher M. (2009). Antioxidants prevent health-promoting effects of physical exercise in humans. Proceedings of the National Academy of Sciences of the United States of America, 106, 8665-8670.
- Bendich A, Machlin LJ. (1988). Safety of oral intake of vitamin E. The American journal of clinical nutrition, 48, 612-619.
- Miller ER, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. (2005). Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Annals of internal medicine, 142, 37-46.
- Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. (2003). Use of antioxidant vitamins for the prevention of cardiovascular disease: metaanalysis of randomised trials. Lancet, 361, 2017-2023.
- Eidelman RS, Hollar D, Hebert PR, Lamas GA, Hennekens CH. (2004). Randomized trials of vitamin E in the treatment and prevention of cardiovascular disease. Archives of internal medicine, 164, 1552-1556.
Tyrosine
Name / Formulation and description:
Tyrosine (TYR) is a dietary non-essential amino acid precursor for catecholamine neurotransmitter synthesis. Tyrosine is contained within protein-rich dietary sources and is synthesised in the liver from phenylalanine.
It is available:
- Commercially in capsules, tablets and water-soluble granules/powder.
- Pharmacological grade (I.e., registered medical nutrition provider) water soluble powder.
Current AIS Supplement Framework Classification: Group B
Agreed AIS Supplement Framework Classification: Group C
- Purported ergogenic effects relative to exercise performance (favouring prolonged endurance), particularly/predominantly in the heat (less so cold and/or hypoxia).
- Purported protection of parameters related to cognitive performance decline relative to exercise performance (favouring prolonged endurance) or stressful exposures, particularly/predominantly in the heat, cold and/or hypoxia.
- Purported protection of parameters related to cognitive performance decline relative to cold exposure.
During prolonged endurance exercise within the heat catecholamine turnover is increased compared to the same exercise in a temperate environment (i.e. the former accelerates fatigue compared to the latter, in part due to central catecholamine depletion of their major precursor TYR).2, 3
During acute stress, there is an observed increase in the activation of noradrenergic neurons in the frontal cortex, which release neurotransmitter as a response to stress.4 The continued release of neurotransmitter is fundamental in the ability to cope with stress, and thus as concentrations begin to deplete, aspects of cognitive function start to deteriorate.5 Therefore, oral supplementation of TYR is proposed to increase its ratio to other large neutral amino acids (LNAA) for competitive transport across the blood brain barrier, thus resulting in a greater cerebral uptake and an increase in dopamine (DA) synthesis in the brain6, 7; i.e. facilitative of prolonging/maintaining ‘optimal’/’minimal’ catecholamine/neurotransmitter presence/function. It is suggested that similar to the effects of physical/exercise/ mental stress and/or heat-stress, catecholamine concentrations also become depleted during exposure to other environmental stressors (e.g. cold and/or hypoxia).8
Physical Performance
Several studies have investigated the effects of TYR in relation to exercise performance in normal9-11 and elevated/ high ambient temperatures.12-15 All three of the studies conducted under temperate conditions failed to observe any beneficial effect of acute TYR ingestion on endurance performance9, 10 or strength and power performance.11 These findings are not surprising due to the questionable amount of stress experienced during exercise in normal ambient temperatures and the relationship between stress and catecholamine turnover.
Recent studies have therefore focused on passive and active heat-stress based designs to examine the influence of TYR under extreme stress. Data has shown significant improvement in exercise capacity (15 ± 11%) after ingestion of 150 mg/ kg body mass of TYR, compared to placebo when cycling to exhaustion in a hot environment (30°C; 50% RH).12 To date, this12 is the first and only study to observe a positive effect of TYR on physical performance, despite the efforts of others13 who attempted to replicate this study. Indeed, others13 reported TYR did not influence exercise capacity or any aspects of cognitive function (reaction time, information processing or memory) in the heat, despite a significant increase in plasma TYR concentration. Indeed, others14 employed a pre-loaded time-trial design based on the theory that a benefit of TYR would be more apparent during self-paced exercise due to the greater influence of behavioural thermoregulation, motivation and arousal compared to constant load exercise.2, 14 However, this was not the case, as TYR ingestion (150 mg/ kg body mass) did not influence time-trial performance when performed in a hot environment (30°C; 50% RH).14 Others15 examined the TYR ingestion (300 mg/ kg body mass) during exposure to a 90 min soccer-simulation protocol [iSPT16] in a warm environment (25°C; 40% RH); TYR had a positive effect on cognitive function (vigilance) and readiness to invest mental effort, but did not influence physical performance.
Cognitive Performance (heat/cold/hypoxia):
The majority of literature assessing the effects of TYR is military based, with several investigations conducted by the US Army Research Institute8, 17-20 and other army institutes.4, 21 These have primarily focused on aspects of cognitive function (complex; working memory, vigilance, tracking and simple reaction time; etc.) and mood during exposure to acute stress, such as such as cold8, 17, 20 and hypoxia17, and paradigms involving both extended wakefulness22 and the physical/emotional stress nexus.21 Each of these aforementioned studies has demonstrated improvements in specific aspects of cognitive function after ingestion of TYR (100-300 mg/ kg body mass; N.B. when 300 mg/ kg body mass of TYR is administered, it is typically via two equal dosages 4 hours apart).
TYR supplementation has direct mechanistic evidence that it can offset heat-induced delays in reaction time during 90 min passive exposure to 45°C; 30% RH.5 This study also assessed higher levels of cognitive function using advanced brain imaging techniques (event related potentials; ERP), providing evidence that heat exposure causes an increase in P300 (reduced concentration) and M100 latency (reduced ability to react to a warning) and
a decrease in M100 amplitude (linked with attention) which returned to near normal levels after ingestion of TYR. It was concluded that the higher DA and norepinephrine (NE) concentrations detected in the TYR trial might have maintained cognitive function by alleviating the decrements associated with heat-stress.5 This5 is the only TYRheat- stress based study to assess DA and NE concentrations in combination with cognitive testing and advanced brain imaging, currently the ‘best’ quality evidence regarding the efficacy of TYR during heat-stress to mediate undesirable heat-mediated cognitive function declines.
There are a number of limitations to the current science. These include:
- A diverse range of administration strategies (dose, form and source of the supplement) reported within the literature.14, 15
- Lack of investigations in the use of TYR in elite athlete populations. In addition, performance tests rarely reflect elite sport competitive events.
- Limited data3, 23 exploring TYR pharmacokinetics in response to pharmacological grade supplementation, limited to dosages of 100 - 300 mg/kg body mass (300 mg/kg body mass dose administered via two equal dosages 4 hours apart).
- Many exercise or ‘stressor’ protocols within the current data may not be ‘stressful’ enough to deplete central catecholamines sufficiently, to surface TYR effects.
- Mechanistic approaches and thus data to quantitatively assess related blood/brain biochemistry changes (including TYR supplementation upon these) alongside advanced brain imaging techniques.
- Not all studies report plasma/serum measures of TYR.
The consensus in available evidence suggests TYR does not have efficacy in improving physical performance (endurance or otherwise; irrelevant of environmental stressor(s)). Conversely to physical performance, TYR does have substantial evidence demonstrating its efficacy to improve aspects of cognitive performance during exposure to heat and/or exercise heat stress, however there have been limited investigations in elite athlete populations.
- Wurtman RJ, Hefti F, Melamed E. (1980). Precursor control of neurotransmitter synthesis. Pharmacological Reviews, 32 (4), 315-35.
- Nybo L, Rasmussen P, Sawka MN. (2014). Performance in the heat-physiological factors of importance for hyperthermia-induced fatigue. Comprehensive Physiology, 4 (2), 657-89.
- Coull N, Chrismas B, Watson P, Horsfall R, Taylor L. (2016). Tyrosine Ingestion and Its Effects on Cognitive and Physical Performance in the Heat. Med Sci Sports Exerc, 48 (2), 277-86.
- Deijen JB, Orlebeke JF. (1994). Effect of tyrosine on cognitive function and blood pressure under stress. Brain Res Bull, 33 (3), 319-23.
- Kishore K, Ray K, Anand JP, Thakur L, Kumar S, Panjwani U. (2013). Tyrosine ameliorates heat induced delay in event related potential P300 and contingent negative variation. Brain and cognition, 83 (3), 324-9.
- Fernstrom JD, Faller DV. (1978). Neutral amino acids in the brain: changes in response to food ingestion. Journal of neurochemistry, 30 (6),1531-8.
- Gibson CJ, Wurtman RJ. (1978). Physiological control of brain norepinephrine synthesis by brain tyrosine concentration. Life Sci, 22 (16),1399-405.
- O’Brien C, Mahoney C, Tharion WJ, Sils IV, Castellani JW. (2007). Dietary tyrosine benefits cognitive and psychomotor performance during body cooling. Physiology & behavior, 90 (2), 301-7.
- Strüder H, Hollmann W, Platen P, Donike M, Gotzmann A, Weber K. (1998). Influence of paroxetine, branched-chain amino acids and tyrosine on neuroendocrine system responses and fatigue in humans. Hormone and metabolic research, 30 (04), 188-94.
- Chinevere TD, Sawyer RD, Creer AR, Conlee RK, Parcell AC. (2002). Effects of L-tyrosine and carbohydrate ingestion on endurance exercise performance. Journal of applied physiology, 93 (5), 1590-7.
- Sutton EE, Coill M, Deuster PA. (2005). Ingestion of tyrosine: effects on endurance, muscle strength, and anaerobic performance. International journal of sport nutrition and exercise metabolism, 15 (2), 173.
- Tumilty L, Davison G, Beckmann M, Thatcher R. (2011). Oral tyrosine supplementation improves exercise capacity in the heat. European journal of applied physiology, 111 (12), 2941-50.
- Watson P, Enever S, Page A, Stockwell J, Maughan RJ. (2012). Tyrosine supplementation does not influence the capacity to perform prolonged exercise in a warm environment. International journal of sport nutrition and exercise metabolism, 22 (5), 363.
- Tumilty L, Davison G, Beckmann M, Thatcher R. (2014). Failure of Oral Tyrosine Supplementation to Improve Exercise Performance in the Heat. Med Sci Sports Exerc, 46 (7), 1417-25.
- Coull NA, Watkins SL, Aldous JW, Warren LK, Chrismas BC, Dascombe B, et al. (2015). Effect of tyrosine ingestion on cognitive and physical performance utilising an intermittent soccer performance test (iSPT) in a warm environment. European journal of applied physiology, 2 (115), 373-86.
- Aldous JW, Akubat I, Chrismas BC, Watkins SL, Mauger AR, Midgley AW, et al. (2014). The reliability and validity of a soccer-specific nonmotorised treadmill simulation (intermittent soccer performance test). Journal of strength and conditioning research / National Strength & Conditioning Association, 28 (7), 1971-80.
- Banderet LE, Lieberman HR. (1989). Treatment with tyrosine, a neurotransmitter precursor, reduces environmental stress in humans. Brain research bulletin, 22 (4), 759-62.
- Lieberman HR. (2003). Nutrition, brain function and cognitive performance. Appetite, 40 (3), 245-54.
- Lieberman HR, Georgelis JH, Maher TJ, Yeghiayan SK. (2005). Tyrosine prevents effects of hyperthermia on behavior and increases norepinephrine. Physiology & behavior, 84 (1), 33-8.
- Mahoney CR, Castellani J, Kramer FM, Young A, Lieberman HR. (2007). Tyrosine supplementation mitigates working memory decrements during cold exposure. Physiology & behavior, 92 (4), 575-82.
- Deijen J, Wientjes C, Vullinghs H, Cloin P, Langefeld J. (1999). Tyrosine improves cognitive performance and reduces blood pressure in cadets after one week of a combat training course. Brain research bulletin, 48 (2), 203-9.
- Neri DF, Wiegmann D, Stanny RR, Shappell SA. (1995). The effects of tyrosine on cognitive performance during extended wakefulness. Aviat space env medi.
- Glaeser BS, Melamed E, Growdon JH, Wurtman RJ. (1979). Elevation of plasma tyrosine after a single oral dose of L-tyrosine. Life sciences, 25 (3), 265-71.